Nanolive Webinar on Phototoxicity
The webinar on Phototoxicity will focus on presenting a recent study realized in collaboration with Ecole Polytechnique Féderale de Lausanne (EPFL). The research compares standard epifluorescence microscopy with the Nanolive label-free tomographic phase imaging technique and discusses the perturbations induced by phototoxicity on living samples during experimentation.
In particular, the following topics will be covered:
- The challenge of observing live cells unperturbed
- The effect of light exposure on living samples
- Nanolive imaging to observe unperturbed organelle movements
Overcoming Phototoxicity - The Nanolive Imaging Advantage
In vitro live cell imaging has become a promising tool to study dynamic cell behaviors, such as division and differentiation, cell death, cell-cell interaction (e.g. immune cells), or cell response to treatments over extended periods of time. However, few technologies have been developed with phototoxicity in mind. Today, most live imaging techniques rely on either high illumination regimes or fluorescent labelling, both inducing phototoxicity and compromising the ability to keep cells unperturbed and alive over time. Since our knowledge of biology is driven by observation, it is key to minimize the perturbations induced by the imaging technique.
Surprisingly, very few studies have highlighted or discussed the artifacts arising from phototoxicity during live cell imaging. This page focuses on the Master Project of Hugo Marc Moreno, which compares standard epifluorescence microscopy with Nanolive imaging (also known as holo-tomographic phase microscopy). Herein, Moreno et al discuss the perturbations induced by phototoxicity on living samples during experimentation.
Nanolive Imaging gets rid of invasive fluorescent markers, while using a low energy exposure light source. Nanolive’s label-free imaging modality makes it well suited to observe living samples, when compared to epifluorescence imaging. Moreno’s et al results raised the question of how much of our knowledge, particularly about mitochondria, may have been biased by artifacts induced by fluorescence imaging.
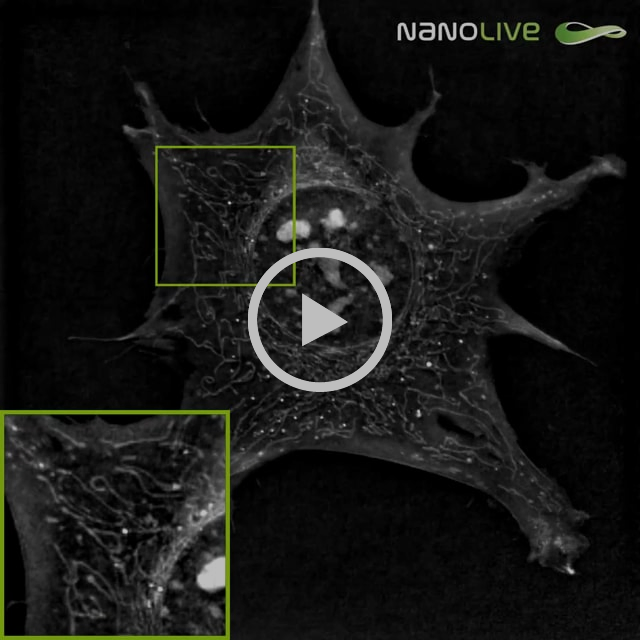
Nanolive microscopy alone did not induce any detectable effect on mouse pre-adipocytes after one-hour acquisition at a frequency of 1 image every 6 seconds. The overall spreading and motility of the cell remained apparently uncompromised: cell shape did not drastically change nor shrink over the imaging period, the ability of filopodia to spread and adhere looked unaffected and main adhesion sites were not impaired. Shape and density of nucleoli (bright granules in the nucleus) did not provide any sign of perturbation neither. Moreover, mitochondrial network did not show any change in phenotype: no extended fusion nor fission was observed, the overall dynamic of the mitochondria remained constant during the full hour of acquisition, and the shape of mitochondria was conserved, no swelling of mitochondria was seen.
Imaging regimen: 1-hour acquisition at a frequency of 1 image every 6 seconds
VS
Cells are affected very rapidly:
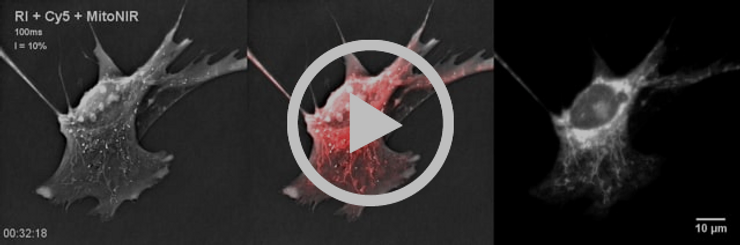
- Appearance of round-shaped or swollen mitochondria was observed with Nanolive technology.
- Loss of mitochondrial specific fluorescent signal and high background or cytosolic fluorescent signal could be observed on the fluorescent image.
- Mitochondrial dynamics slowed down and reached an almost complete stop after 38 minutes of exposure.
- Very slow cell shrinkage was observed until timepoint 00:58:00, at which a consequent proportion of the cell lost adherence.
MitoNIR signal was concentrated in the nuclear periphery at later timepoints.
If you are interested in learning more, look into Moreno’s et al. Master thesis here.
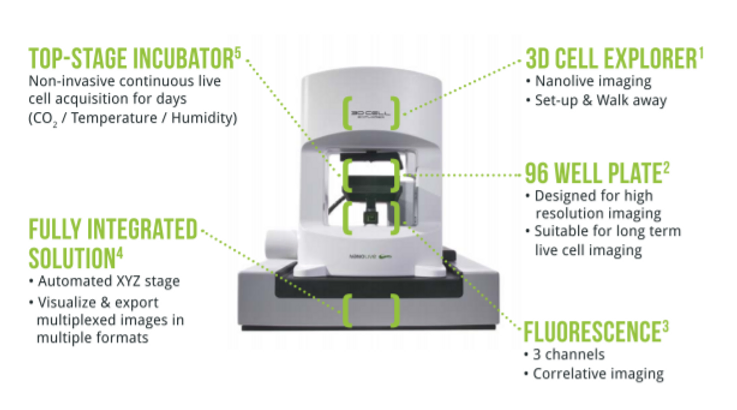
The Latest in Nanolive Imaging: CX-A
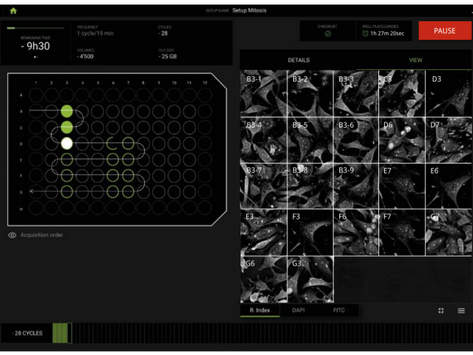
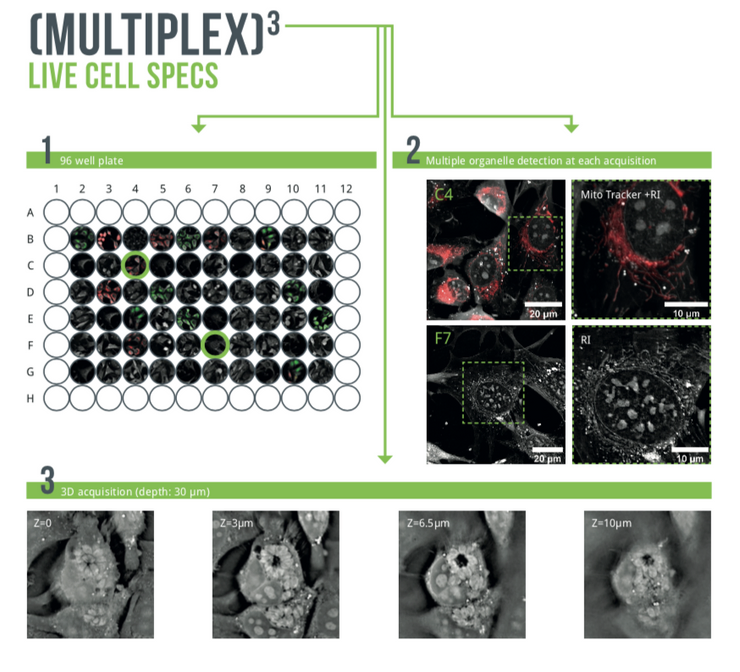
The CX-A redefines the limits of live cell imaging in 96 well plates for continuous organelle monitoring in cell populations.

Complete Solution for 3D Live Cell Exploration
The CX-A is designed to work with 96 well plates to multiply and parallelize experimental conditions, hence, bringing undoubtable significance to each experiment and delivering solid biological insights to researchers (1).
Furthermore, the system is equipped with multiple imaging modalities to correlate and compare physical and chemical information at each time-point (2).
Finally, 3D data sets of every single image at every single time-point are automatically acquired in real-time (3).
A fully integrated solution adapted to the most advanced professional needs. Click here for a full overview of applications.
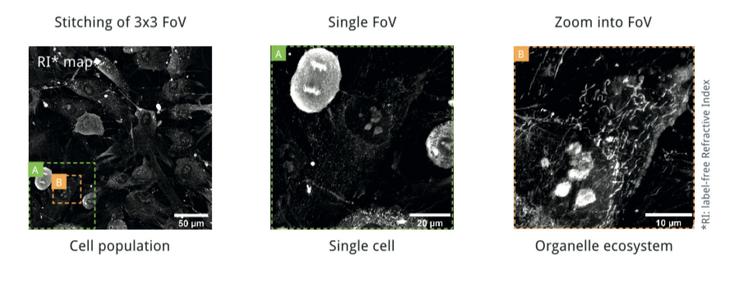
Observe Living Cells - From Populations to Organelles